
In the pediatric population, pain is frequently under-recognized and inadequately treated. Improved education and training of health care providers can positively impact the management of pain in children. The purpose of this review is to provide a practical clinical approach to the management of acute pain in the pediatric inpatient population. This will include an overview of commonly used pain management modalities and their potential pitfalls. For institutions that have a pediatric acute pain service or are considering initiating one, it is our hope to provide a useful tool to aid clinicians in the safe and effective treatment of pain in children.
Avoid common mistakes on your manuscript.
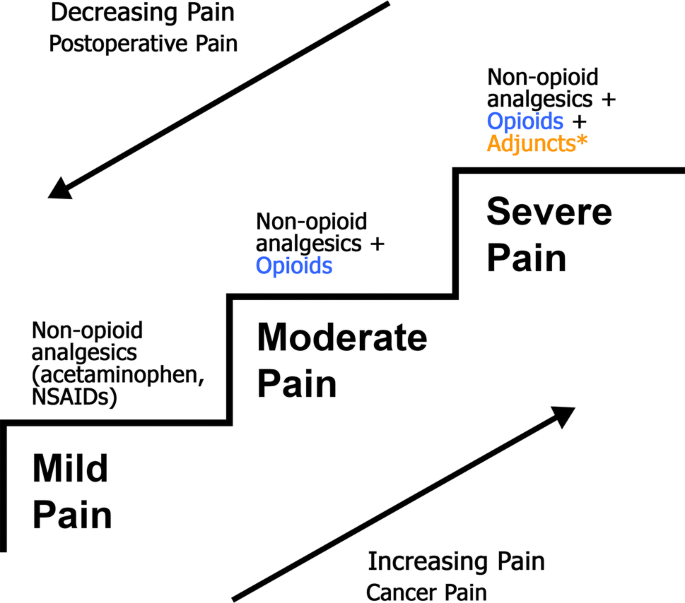
Effective pain management is ideally practiced in a multidisciplinary model focusing on patient-centered care. The World Health Organization (WHO) [1] analgesic ladder provides a strong foundation for the treatment of pain that can be built upon to reflect more modern thinking and techniques around pain management (Fig. 1). Some of these modifications are presented in an updated WHO ladder with guiding principles on post-operative management of acute pain [2], which advocates 5 recommendations for the correct use of analgesics: (1) use the oral form of medication whenever possible, (2) analgesics should be given at regular intervals, (3) analgesics should be administered based on the severity of pain assessed using a pain intensity scale, (4) medication dosing should be tailored to the individual patient, and (5) attention to detail should be maintained throughout the prescription of pain medications.
The acute pain service (APS) is a specialized, multi-disciplinary inpatient team consulted to assist with management of severe pain. Within our institution, this team consists of a pediatric anesthesiologist, pediatric anesthesia fellow, clinical nurse specialist, and pediatric psychiatrist. The APS works in collaboration with the patient’s primary care team, bedside nurse, family, and pharmacists to provide a patient-centered multi-modal pain plan. Generally, the APS is consulted to assist in pain management when either a patient’s analgesic needs have grown beyond standard opioid dosing (Table 1) that their primary service is comfortable prescribing, or there is anticipated need for APS involvement for postoperative patients. Postoperative patients who automatically require APS management in our institution include those with an indwelling regional or neuraxial block catheter, patients who have received a single-shot peripheral nerve block, patients with a patient-controlled analgesia (PCA) technique, or patients receiving a Ketamine infusion.
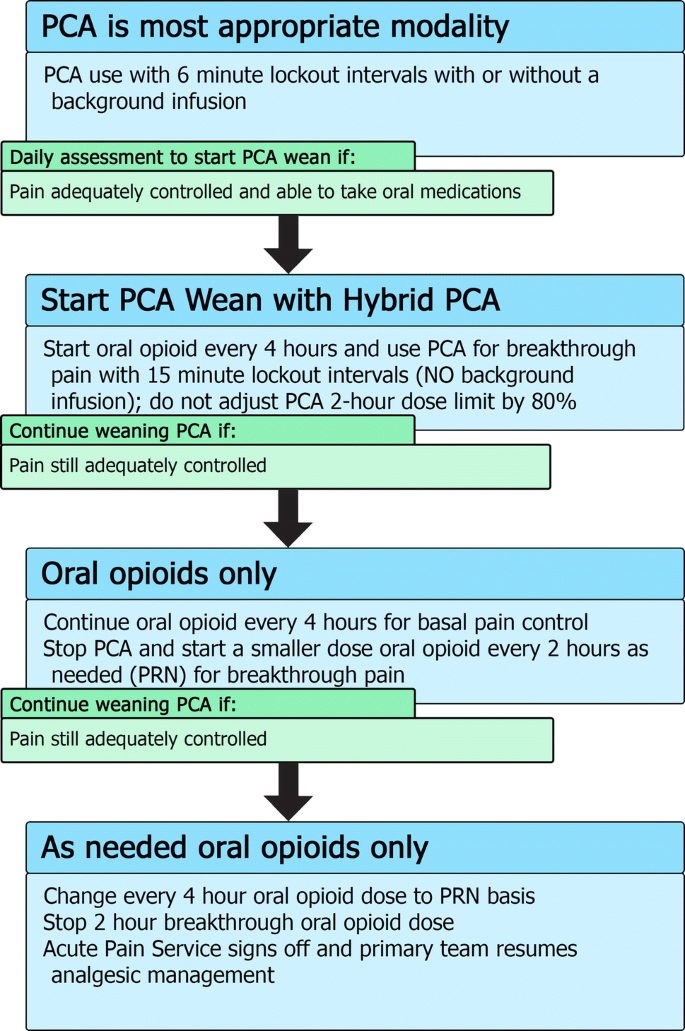
For patients who continue to require opioids and are able to tolerate oral intake and, thus, can be treated with oral medications, we use a “Hybrid PCA” modality that combines oral medications with PCA for breakthrough pain in the process of weaning a patient off their PCA (Fig. 2). This allows patients the ability to continue to titrate their opioid usage to their pain while at the same time starting them on an oral form of opioid to allow more stable plasma concentrations of drug (see Table 1 for conversion doses).
For hybrid PCA dosing, the same bolus dose can be used but with an increase of the lockout interval to 15 min. There is no background PCA infusion as the basal opioid intake should be in the form of the oral medication. The 2-h maximum dose will be 100% of the 2-h calculated maximum dose (rather than adjusted to 80%). If, after a day of the hybrid PCA, the patient’s pain is still well controlled, the PCA is stopped completely and the breakthrough dosing is converted to an oral opioid dose on an as-needed basis. On the next day, if the pain is still well controlled with little use of breakthrough doses, the regularly dosed opioid can be converted to an as-needed basis only. Typically, most pediatric services are then able to manage such an oral opioid regimen and the APS signs off.
For a typical case example in the APS management of a PCA, please see Online Resource 2.
Epidural analgesia is a safe and effective method of managing pain in children [38,39,40]. Currently, the literature surrounding epidural use in children is lacking when compared with adult studies. In adults, numerous trials have shown superior pain relief when comparing epidural techniques to oral or intravenous opioids, or other alternative approaches [12, 41,42,43,44,45]. In abdominal surgery, epidural local anesthetic hastens the return of bowel function and decreases postoperative pain [46].
The focus of this review is not to discuss epidural insertion techniques, but instead to discuss managing epidural catheters and infusions on the acute pain service.
At our institution, the available solutions for continuous regional block infusions are all bupivacaine based with the options to add epinephrine and/or fentanyl. Table 5 summarizes the typical starting doses for epidural infusions within our institution. Alternative local anesthetics include ropivacaine and levobupivacaine, both of which are less cardiotoxic than racemic bupivacaine [47]. We currently do not offer patient-controlled epidural analgesia. Generally, most patients are started on epidural infusions using 0.1% bupivacaine with epinephrine, with more dilute concentrations used initially if there is concern from surgeons about being able to accurately assess neurologic function distally. The addition of an epidural opioid is not as standardized and is decided on a case-by-case basis. Some APS physicians choose not to add opioid into the epidural solution if there will be a second administration source of opioid, as this can confound the ability to titrate opioids with two concurrent sources. This second source may be in the form of intrathecal, oral, or systemic opioids. Two simultaneous sources of opioids may increase the risk of opioid side effects, making it more challenging to titrate to desired clinical effect.
For a typical case example in the APS management of an epidural, please see Online Resource 3.
Typically, peripheral nerve blockade is performed in our center as single-shot injections with occasional placement of peripheral nerve catheters. Any peripheral nerve block that has a motor block component will be followed by the APS until the day after block performance or nerve catheter removal. This is to ensure satisfactory recovery of nerve function as well as a smooth transition to oral pain medications. For procedures where severe pain is anticipated in the first day postoperatively with a single-shot block, regularly scheduled oral opioids can be initiated at 6–8 h post-peripheral nerve blockade to ensure loading of oral opioid prior to the nerve block wearing off.
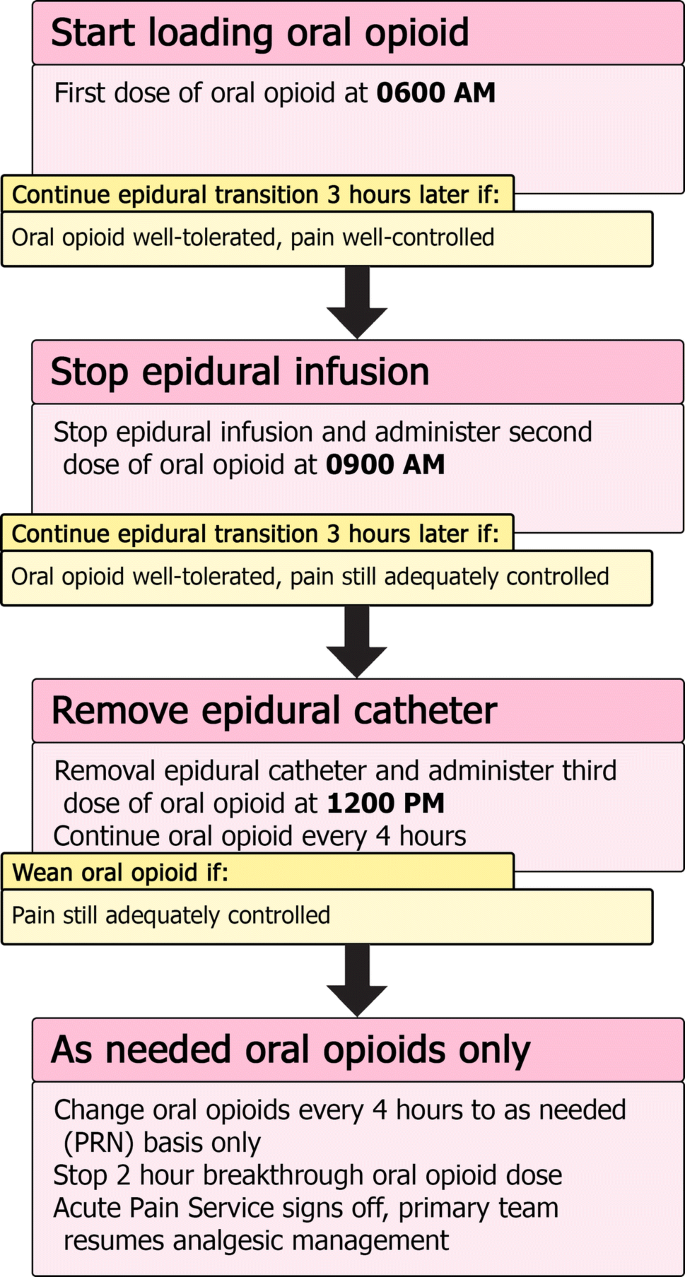
For indwelling nerve catheters, much of the same principles in management of epidural catheters can be applied, with daily APS visits to ensure ongoing effective pain management, minimal side effects, and to assess the catheter insertion site for signs of infection or other concerning features. When planning the removal of a nerve block catheter with anticipation of ongoing significant pain, the same general approach of administering doses of oral opioid prior to stopping an epidural infusion can be applied to transitioning from a nerve block catheter (Fig. 3).
For institutions considering the implementation of a new APS, we will describe the experience in our institution and issues to consider when planning and growing such a service. Initial planning should involve gathering data on current cases (i.e., major surgical operations) that might require APS management to assess whether there is the caseload to justify establishment of an APS. Furthermore, a survey of patients, families, and health care professionals involved in the postoperative care of patients undergoing procedures at high risk for postoperative pain should be undertaken to establish the current adequacy of pain management and look for need for an APS.
Once it is decided that there is a sufficient demand for an APS, a dedicated anesthesiologist should lead the team in establishing the APS within the hospital. It will be important to meet with hospital administrators to ensure their support of the establishment of an APS. A nurse with an interest in pain management should be involved, ideally with a background in care for post-operative patients, and be ready to advocate for the APS throughout the hospital and with nursing colleagues. More training should not be a mandatory requirement at the beginning, since much of the training can come from experience and working with the anesthesiology department. Clinically, there should be 24-h coverage of the APS by anesthesiologist services, but this does not necessarily need to be a dedicated APS anesthesiologist especially at nighttime and over weekends.
Nurse-based APS models are well established [48, 49], and may be a good cost-effective starting point, in which a dedicated pain nurse staffs APS during the daytime, with supervision from an anesthesiologist. The pain nurse plays a significant role in managing APS modalities and educating ward nurses and other staff around APS issues [50]. Overnight and weekend coverage can include a second nurse or anesthesiology trainee under supervision by a consultant anesthesiologist.
Foundational work should include multi-disciplinary discussion and writing of pain-related policies, such as minimum safety monitoring for various pain modalities (Online Resource 4). The equipment required will be that involved in placement of peripheral or neuraxial nerve blocks, and any infusion pumps to maintain continuous infusions. We would recommend the establishment of a registry or database for continuous quality improvement.
Once the groundwork has been established, and all the safety mechanisms and policies have been finalized, the clinical implementation of the APS should be launched in a stepwise approach, with a pilot program in a single patient population of similar surgical procedures. It would also be safer to initially start the program in older children. Ongoing quality improvement strategies such as a repeat survey to assess for improved pain control after APS implementation will help the service improve and expand to more patient population groups, and eventually throughout the hospital.
Although there will be costs associated with an APS service, there should not be great additional costs other than the aforementioned specialist time and some basic equipment for running of modalities such as PCA or nerve block infusions. One center estimated establishing APS in their institution at a cost of $3–4 USD per patient [49], however this did not include equipment costs. An effective APS could contribute to improved pain control, improved patient and family satisfaction, decrease opioid consumption, and decrease adverse effects associated with uncontrolled severe pain [51,52,53,54]. In our center, we also provide a significant educational role to nurses and medical services in multi-modal pain management. It has been argued that an effective APS can offer cost-savings [53, 55, 56], but given the multi-factorial effects and considerations involved with running an APS, this is hard to definitively determine.
In areas of low resource, pain management could be greatly improved with increased education, awareness, and training of practitioners in pain medicine [57, 58]. In such settings, a model that would likely be successful is improvement in the recognition and treatment of pain with standard modalities, such as morphine, rather than new techniques.
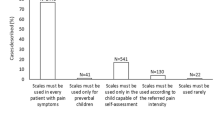
There is ongoing need for improvement in the management of acute pain in hospitalized pediatric patients. Future research and quality improvement could benefit from focusing on ensuring adequate training and standardization of pain assessment for all health care providers. Hospital protocols should include an assessment of pain with every nursing shift for each patient with clear documentation to allow for assessment of trends. Ongoing training in pain management should be implemented for all physicians who treat children. The treatment of pain in children should not be the sole focus of physicians on the pain service but of any health care providers who care for children. It would also be very valuable if a cost–benefit analysis of APS care could be measured, and this could give institutions who do not yet support an APS more reliable information with which to make future decisions.
For a variety of reasons, pediatric pain is still under-treated in modern medicine. One identified problem has been limited experience and training in acute pain management for physicians treating children. It is our hope that by describing typical practices used in our institution, we can share our knowledge and experience with other professionals endeavoring to safely minimize painful experiences for children.
A part of the contents of this manuscript was presented in the 62nd Annual Meeting of The Japanese Society of Anesthesiologists, Kobe, Japan, and the 22nd Annual Meeting of the Japanese Society of Pediatric Anesthesiology, Yokohama, Japan (KA). All authors wish to thank and acknowledge colleagues in the Acute Pain Service (Anesthesia and Pain Medicine, The Hospital for Sick Children, University of Toronto) for their contribution to conceiving this article.